News
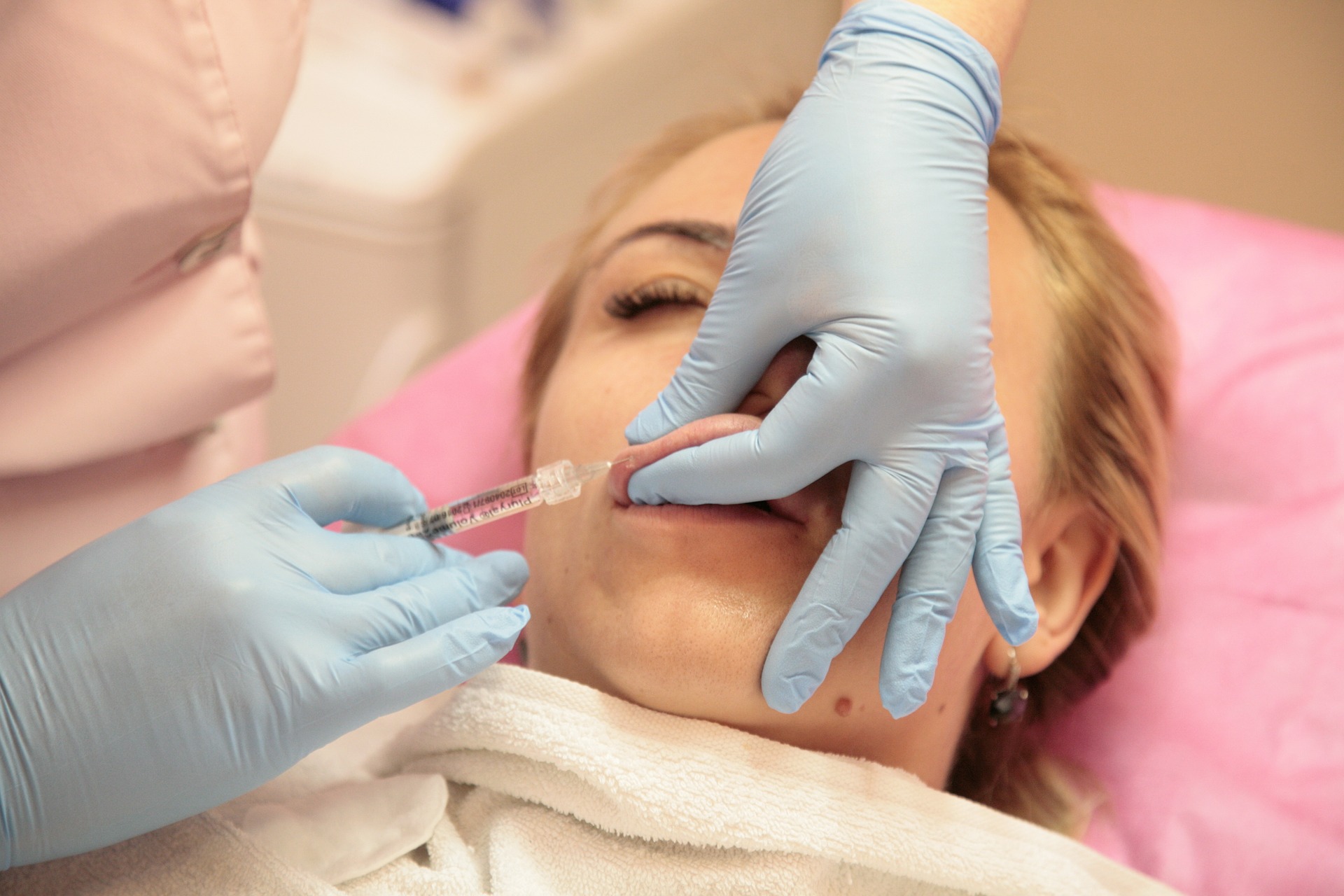
Law is published that regulates the use of modeling substances in body treatments for aesthetic purposes and defines said procedure as a medical act

El Congreso de la República de Perú a través de la Ley Nº 31014, publicada el 28 de marzo del 2020 en el Diario Oficial “El Peruano”, dispuso la publicación de la Ley que regula el uso de sustancias modelantes en tratamientos corporales con fines estéticos y define dicho procedimiento como acto médico.
El objetivo de la citada ley es proteger la vida y la integridad física y psicológica de los ciudadanos, a través de la regulación del uso de sustancias modelantes en tratamientos corporales con fines estéticos.
Por cuanto se mencionan a continuación algunas de las disposiciones establecidas a través de esta ley:
- En base a que los tratamientos que empleen las mencionadas sustancias son un acto médico, estos tratamientos deben realizarse en un establecimiento de salud, y éste deberá ser calificado por el Ministerio de Salud.
- La DIGEMID será quien brinde la propuesta de listado de sustancias modelantes reguladas, con el sustento técnico correspondiente al Ministerio de Salud.
- Así también la DIGEMID será quien tenga la función de vigilancia, fiscalización y cumplimiento de la citada ley; y será la facultada a efectuar la cancelación de los registros sanitarios de todas aquellas mezclas de biolólimeros inyectables o sustancias de relleno inyectables no autorizadas en el listado que aprobara el Ministerio de Salud.
Source: Official Gazette "El Peruano"
Recent posts

Extension of the declaration of Sanitary Emergency

The MINSA included in the PNUME for the Health Sector, the product Medicinal Oxygen 93% gas for inhalation

MINSA ordered the publication of the Regulation Project that establishes specific provisions for conducting Clinical Trials with Medical Devices

The MINSA approved the Sanitary Directive for the use of Oxygen in Institutions Providers of Health Services and home use

MINSA approved the Technical Document: National Vaccination Plan against COVID-19

The MINSA orders the publication of the Technical Document project: Manual of Citizen Participation in the elaboration of the PNUME

MINSA approved the Technical Document: Management of people affected by COVID-19 in hospitalization services

MINSA approved the technical document: Guidelines for the exceptional health authorization of medical devices in the research stage

Urgent decree that dictates extraordinary measures to facilitate and guarantee the acquisition, conservation and distribution of vaccines against COVID-19

The MINSA plans to publish a draft ministerial resolution that resolves to include in the PNUME for the health sector, the medicinal oxygen product 93% gas

DIGEMID safety modifications: For pharmaceutical specialties containing "MELOXICAM"

The MINSA approves the update of the scale for infractions and sanctions to pharmaceutical and non-pharmaceutical establishments of the regulation of pharmaceutical establishments

DIGEMID safety modifications: For pharmaceutical products containing "Bacillus Clausii"

DIGEMID safety modifications: For pharmaceutical specialties containing "MONTELUKAST"

DIGEMID safety modifications: For pharmaceutical specialties containing "LORCASERIN"

DIGEMID safety modifications: For pharmaceutical specialties that contain "FENOTEROL"

The MINSA modified the Regulation of Narcotic Drugs, Psychotropic Drugs and Other Substances subject to Sanitary Inspection

The MINSA approved the "Technical Health Standard that establishes the ethical criteria for the promotion and advertising of pharmaceutical products, medical devices and health products"

Health Directive for the Control and Surveillance of in vitro Diagnostic Devices is approved: Rapid and Molecular Tests for COVID-19