The healthcare industry consists of a variety of products and devices intended to promote wellness and treat disease. Among these, two main categories stand out for their importance and function: Pharmaceutical products and medical devices. Although both are designed to improve people’s health and quality of life, there are fundamental differences that distinguish them in terms of composition, function, regulation and clinical application. Which ones are we talking about? That’s something you’ll discover in the following article.
Pharmaceuticals products and medical devices
Understanding better its concept, beyond pharmaceuticals, all kinds of herbs or medical supplements that fulfill the role of prevention and treatment of ailments to the general public would also enter the list. Its relevance is such that until 2017, the National Institute of Statistics and Informatics (INEI), working together with the Ministry of Production, recorded in its publication «Sector Research Study» a total of 196 manufacturing companies concentrated in Lima. This is attributed to 84% compared to the rest of the regions.
The same document also detailed that pharmaceutical products have a higher rate of demand when purchased by families (households), reaching 62.4%, followed by the public health sector (hospitals, health centers, clinics) with 13.1%. These figures reveal that it is the people themselves who seek medicines regardless of whether or not they have a doctor’s order. Such is the case that today the pharmaceutical industry is considered «high-tech manufacturing» according to the United Nations Industrial Development Organization.
And medical device?
Medical devices are instruments, apparatus, equipment or materials used for diagnosis, monitoring, treatment or rehabilitation of diseases or disabilities. They can range from simple diagnostic tools, such as stethoscopes and thermometers, to more complex devices, such as cardiac pacemakers and joint replacements. They are often designed to interact directly with the human body or facilitate the delivery of medical treatments.
Sanitary Controls in Perú
In Peru, it is the DIGEMID who is in charge of regulating and granting certifications to those drugstores and factories that seek to distribute their products in national territory (regardless of whether or not their origin is Peruvian). Therefore, its main function is to ensure that the population has access to quality drugs and to ensure that they are used rationally, as typified by Law No. 584 published in 1990. But: How does it achieve this?
This is possible because they carry out a previous evaluation of the merchandise taking as a reference the technical information that guarantees its safety, efficacy and quality. In this way, they monitor all aspects related to the use and distribution of pharmaceutical products through a label description. However, this is not enough since they only operate under a sample with a high margin of error, which is when pharmacovigilance strategies come into play to detect possible cases of adverse reactions, lack of efficacy or abuse.
Which products do not require sanitary registrations?
Products that do not require sanitary registrations are those that either comply with current regulations and are under international technical standards, or that on the contrary have very particular characteristics that exempt them from any procedure. In 2021, MINSA updated the official list of such goods, keeping several others in force, such as anticorrosives and accessories for printers and exercise equipment. In the document, which includes a total of 1094 supplies, the following are highlighted:
- Hospital furniture.
- Reusable gowns for patients.
- Chemical products and reagents.
- Dental supplies and dental laboratory equipment.
- Biomedical equipment and supplies for clinical laboratory use,
- Others.
So how do they differ?
The fundamental differences between pharmaceutical products and medical devices are also reflected in their function and clinical application. Drugs are intended to alter the physiology of the human body at the cellular or molecular level, either by inhibiting the activity of certain enzymes, blocking specific receptors or supplementing biochemical deficiencies. Their primary purpose is to treat existing diseases, alleviate symptoms or prevent the progression of medical conditions.
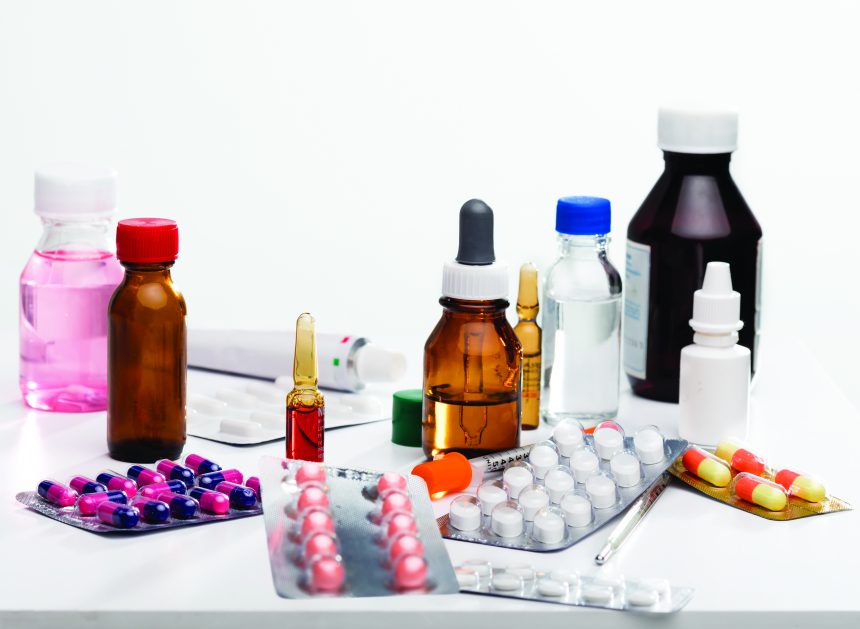
On the other hand, medical devices often have a more direct, physical focus on diagnosing, treating or monitoring health conditions. For example, imaging devices, such as MRI and CT scans, are used to visualize anatomical structures and detect abnormalities or injuries. Implantable devices, such as pacemakers and prostheses, help restore physical function compromised by disease or injury.
Benefits of pharmaceutical products
- Treatment of diseases. The most relevant benefit of these products is that they are essential for the treatment of acute and chronic diseases. They contribute significantly to the management and cure of various health conditions.
- Prolongation of life. Many drugs and treatments have proven to be effective in prolonging life and improving survival in patients with serious diseases, such as cancer. Advances in pharmaceutical research have led to innovative therapies that have changed the prognosis of diseases previously considered incurable.
- Continuous development. One fact that cannot be overlooked is that the pharmaceutical industry is constantly engaged in research and development of new drugs. This continuous innovation leads to the introduction of more effective therapies, less invasive treatments and drugs with fewer side effects, thus improving the efficacy and tolerability of treatments for the benefit of the community.
Contact SCR Consultores
Not all companies are trained to know how to handle the sanitary registrations of their drugs, and that incurs more expenses in time and money. But that is not a problem for SCR Consultores. With international recognition in countries like Bolivia, our work prioritizes client convenience with immediate answers and solutions. This fact led us to partner with the Lima Chamber of Commerce (CCL), the health guild (COMSALUD) and the Bolivarian National Chamber of Commerce (CNC) to strengthen the industry.
Therefore, the best way to know which products must pass this filter is through the personalized advice that our professional technical team will provide you from the first contact. Find out all the requirements to register your pharmaceutical products by Clicking Here. You can also call them at (511) 711-9070 or send us an email to jludena@scrconsultores.com.pe. Find us at Calle Rio de la Plata N ° 305 – 315, San Isidro. Lima, Peru. SCR Consultants, guiding the way of the national industry.